For patients with 1L r/m NSCLC (PD-L1 <1% and PD-L1 ≥1%)1†
Reevaluate your current treatment approach

Featuring unprecedented 6-year OS follow-up data.a Give patients with PD-L1 <1% a chance for long-term, durable survival with OPDIVO + YERVOY and 2 cycles of chemo.1,2b‡
aBased on a minimum/median follow-up of 68.6/75.8 months in Checkmate 9LA.2
bExploratory analysis; study was not powered for comparison.2
OPDIVO (10 mg/mL) and YERVOY (5 mg/mL) are injections for intravenous use.1,3
*Platinum-doublet chemotherapy.
†Without EGFR and ALK aberrations.1
‡In Checkmate 9LA, patients received 2 cycles of platinum-doublet chemo q3w in the experimental arm and 4 cycles in the comparator arm; NSQ: pemetrexed + carboplatin or cisplatin (optional pemetrexed maintenance therapy in the comparator arm only); SQ: paclitaxel + carboplatin.1
1L=first-line; ALK=anaplastic lymphoma kinase; CI=confidence interval; EGFR=epidermal growth factor receptor; HR=hazard ratio; ITT=intent to treat; mNSCLC=metastatic non-small cell lung cancer; NSCLC=non-small cell lung cancer; OS=overall survival; PD-L1=programmed death-ligand 1.
INDICATION OPDIVO® (nivolumab), in combination with YERVOY® (ipilimumab) and 2 cycles of platinum-doublet chemotherapy, is indicated for the first-line treatment of adult patients with metastatic or recurrent non-small cell lung cancer (NSCLC), with no EGFR or ALK genomic tumor aberrations.
CHECKMATE 9LA: FOR PATIENTS WITH r/m NSCLC (PD-L1 <1% and PD-L1 ≥1%)
Unprecedented survival rate in patients with PD-L1 <1%: The only I-O combination with 20% of patients alive at 6 years1,2
OVERALL SURVIVAL IN PD-L1 <1%: EXTENDED FOLLOW-UP ANALYSIS AT 6 YEARS2,4,5

Minimum/median follow-up for OS: 68.6/75.8 months.2
Limitation: Checkmate 9LA was not powered to detect differences in the treatment effect in PD-L1 subgroups; therefore, results from this exploratory analysis should be interpreted with caution because of the limited patient numbers and potential imbalances in baseline characteristics within the subgroup.2
- At the initial pre-specified interim analysis in the ITT population at the 8.1-month minimum follow-up, the median OS was 14.1 months (95% CI: 13.2–16.2) with OPDIVO + YERVOY with chemo and 10.7 months (95% CI: 9.5–12.5) with chemo alone; HR=0.69 (96.71% CI: 0.55–0.87); P=0.00061,4
- At the 68.6-month minimum follow-up analysis in the ITT population, median OS was 15.8 months (95% CI: 13.9–19.7) with OPDIVO + YERVOY with chemo and 11.0 months (95% CI: 9.5–12.7) with chemo; HR=0.74 (95% CI: 0.63–0.87)2
- At the 68.6-month minimum follow-up analysis in patients with PD-L1 <1%, median OS was 17.7 months (95% CI: 13.7–20.3) with OPDIVO + YERVOY with chemo and 9.8 months (95% CI: 7.7–13.5) with chemo; HR=0.64 (95% CI: 0.49–0.84)2
- At the 68.6-month minimum follow-up analysis in patients with PD-L1 ≥1%, 15% were still alive in the OPDIVO + YERVOY with chemo arm (median OS of 15.8 months [95% CI: 13.8–22.2]) compared with 10% in the chemo arm (median OS of 10.9 months [95% CI: 9.5–13.2]); HR=0.75 (95% CI: 0.61–0.92)2
Study design: Checkmate 9LA was a randomized (1:1), open-label, phase 3 study of OPDIVO 360 mg q3w in combination with YERVOY 1 mg/kg q6w and 2 cycles of histology-based chemotherapy vs 4 cycles of platinum-doublet chemotherapy* as a first-line treatment in patients with metastatic or recurrent NSCLC regardless of histology or PD-L1 status. Key eligibility criteria included patients 18 years or older, stage IV or recurrent NSCLC, ECOG PS 0/1 and no prior systemic anticancer therapy. Patients with known EGFR mutations or ALK translocations sensitive to available targeted inhibitor therapy, untreated brain metastases, carcinomatous meningitis, active autoimmune disease, or medical conditions requiring systemic immunosuppression were excluded from the study. Treatment continued until disease progression, unacceptable toxicity, or for up to 2 years. Patients were stratified by histology (SQ vs NSQ), PD-L1 (<1% vs ≥1%), and sex. The primary endpoint was OS. Additional efficacy outcome measures were PFS, ORR, and DOR.1,6
*In Checkmate 9LA, patients received 2 cycles of platinum-doublet chemo q3w in the experimental arm, and 4 cycles in the comparator arm; NSQ: pemetrexed + carboplatin or cisplatin (optional pemetrexed maintenance therapy in the comparator arm only); SQ: paclitaxel + carboplatin.1
DOR=duration of response; ECOG=Eastern Cooperative Oncology Group performance status; mos=months; NR=not reached; NSQ=non-squamous; ORR=overall response rate; PFS=progression-free survival; q3w=every 3 weeks; q6w=every 6 weeks; r/m NSCLC=recurrent or metastatic non-small cell lung cancer; SQ=squamous.
Early separation* observed and durable survival at 6 years with OPDIVO + YERVOY and 2 cycles of chemo vs chemo alone1,2†‡
OVERALL SURVIVAL ITT POPULATION (EXTENDED FOLLOW-UP ANALYSIS AT 6 YEARS)2,4,5

*Early separation of the curves is observational and not powered to detect differences in the treatment effect.3
Minimum/median follow-up for OS: 68.6/75.8 months.2
- Efficacy results from the pre-specified interim analysis when 351 events were observed (87% of the planned number of events for final analysis) with an 8.1-month minimum follow-up1,4
- At the 68.6-month minimum follow-up in the ITT population, 19% of patients were still in response in the OPDIVO + YERVOY with chemo arm (median DOR of 13.0 months [95% CI: 8.7–20.2]). All patients with continuing response in the chemo arm were censored prior to 72 months, so response rate is not available (median DOR of 5.6 months [95% CI: 4.4–7.1])2
- Median OS in the ITT population at the 68.6-month minimum follow-up analysis was 15.8 months (95% CI: 13.9–19.7) with OPDIVO + YERVOY with chemo and 11.0 months (95% CI: 9.5–12.7) with chemo; HR=0.74 (95% CI: 0.63–0.87)2
- Median PFS in the ITT population at the 68.6-month minimum follow-up was 6.7 months (95% CI: 5.6–8.0) with OPDIVO + YERVOY with chemo and 5.3 months (95% CI: 4.4–5.6) with chemo; HR=0.70 (95% CI: 0.59–0.82)2
- ORR in the ITT population at the 68.6-month minimum follow-up was 38% (95% CI: 33–43) with OPDIVO + YERVOY with chemo and 25% (95% CI: 21–30) with chemo2,6
- 37% of patients enrolled had PD-L1 expression of <1%; 24% had PD-L1 expression of ≥50%7
- 32% of patients enrolled had SQ disease; 68% had NSQ disease1
†In Checkmate 9LA, patients received 2 cycles of platinum-doublet chemo q3w in the experimental arm, and 4 cycles in the comparator arm; NSQ: pemetrexed + carboplatin or cisplatin (optional pemetrexed maintenance therapy in the comparator arm only); SQ: paclitaxel + carboplatin.1
‡In the intent-to-treat population vs chemo.1
Select Important Safety Information
Serious Adverse Reactions
In Checkmate 9LA, serious adverse reactions occurred in 57% of patients (n=358). The most frequent (>2%) serious adverse reactions were pneumonia, diarrhea, febrile neutropenia, anemia, acute kidney injury, musculoskeletal pain, dyspnea, pneumonitis, and respiratory failure. Fatal adverse reactions occurred in 7 (2%) patients, and included hepatic toxicity, acute renal failure, sepsis, pneumonitis, diarrhea with hypokalemia, and massive hemoptysis in the setting of thrombocytopenia.
Common Adverse Reactions
In Checkmate 9LA, the most common (>20%) adverse reactions were fatigue (49%), musculoskeletal pain (39%), nausea (32%), diarrhea (31%), rash (30%), decreased appetite (28%), constipation (21%), and pruritus (21%).
Please see additional Important Safety Information below.
At 6 years, 25% of patients with PD-L1 <1% were still in response after being off protocol therapy ≥4 years2*†
DURATION OF RESPONSE IN PD-L1 <1%: EXTENDED FOLLOW-UP ANALYSIS AT 6 YEARS2

Minimum/median follow-up for OS: 68.6/75.8 months.2
Limitation: Checkmate 9LA was not powered to detect the differences in the treatment effect in this subgroup; therefore, this exploratory analysis should be interpreted with caution because of the limited patient numbers and potential imbalances in baseline characteristics within the subgroup.
- At the 68.6-month minimum follow-up in the ITT population, 19% of patients were still in response in the OPDIVO + YERVOY with chemo arm (median DOR 13.0 months [95% CI: 8.7–20.2]). All patients with continuing response in the chemo arm were censored prior to 72 months, so response rate is not available (median DOR 5.6 months [95% CI: 4.4–7.1])2
- At the 68.6-month minimum follow-up in patients with PD-L1 ≥1%, 16% of patients were still in response in the OPDIVO + YERVOY with chemo arm (median DOR 11.8 months [95% CI: 8.6–20.3]). All patients with continuing response in the chemo arm were censored prior to 72 months, so response rate is not available (median DOR 5.6 months [95% CI: 4.3–8.0])2
- In the ITT population at the 68.6-month minimum follow-up, ORR was 38% (137/361) with OPDIVO + YERVOY with chemo and 25% (90/358) with chemo2
- At the 68.6-month minimum follow-up in patients with PD-L1 <1%, ORR was 31% (42/135) with OPDIVO + YERVOY with chemo and 20% (26/129) with chemo2
- In patients with PD-L1 ≥1% at the 68.6-month minimum follow-up, ORR was 43% (87/204) with OPDIVO + YERVOY with chemo and 28% (56/204) with chemo2
*In Checkmate 9LA, the primary efficacy outcome measure was OS. Additional efficacy outcome measures included ORR and DOR.1
†38% vs 50% of patients in the respective arms received subsequent therapy.2
NCCN recommendations for patients with metastatic NSCLC8
Nivolumab (OPDIVO) + ipilimumab (YERVOY) + platinum-doublet chemotherapy* PD-L1 <1% and PD-L1 ≥1%
NCCN Category 1, other recommended
- Nivolumab (OPDIVO) + ipilimumab (YERVOY) + platinum-doublet chemotherapy* is recommended as a Category 1, other recommended, first-line therapy option for eligible patients with metastatic NSCLC regardless of PD-L1 expression and performance status 0–1 (PD-L1 <1%) or 0–2 (PD-L1 ≥1%) (V.3.2025), who are EGFR,† ALK, ROS1, BRAF V600E, NTRK1/2/3, METex14, and RET negative, and with no contraindications to PD-1 or PD-L1 inhibitors8
*Histology-based chemotherapy; NSQ: pemetrexed + (carboplatin or cisplatin); SQ: paclitaxel + carboplatin.1
†EGFR exon 19 deletion, exon 21 L858R, EGFR S7681, L861Q, and/or G719X mutation negative.8
Please see updated NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for a complete listing of all NCCN-recommended agents, including preferred options. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
NCCN=National Comprehensive Cancer Network® (NCCN®); NTRK=neurotrophic tyrosine receptor kinase; PD-1=programmed cell death protein-1.
OPDIVO + low-dose YERVOY (1 mg/kg) and 2 cycles of chemo1*†
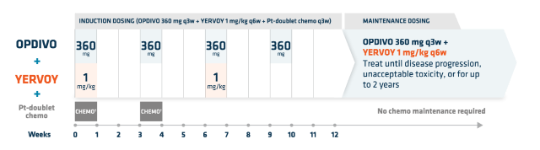
- OPDIVO is administered as an IV infusion over 30 minutes1
- YERVOY is administered as an IV infusion over 30 minutes3
*For the r/m NSCLC dosing regimen in combination with chemo: on the first week, 4 agents will be administered (OPDIVO 360 mg + YERVOY 1 mg/kg + histology-based† chemo), followed by 3 agents (OPDIVO + histology-based† chemo) on the third week, 2 agents (OPDIVO + YERVOY) on the sixth week, and OPDIVO monotherapy on the ninth week, followed by maintenance therapy of OPDIVO + YERVOY.1
†Histology-based chemo: SQ patients: carboplatin AUC 6 + paclitaxel 200 mg/m2 q3w; NSQ patients: carboplatin AUC 5 or 6 + pemetrexed 500 mg/m2 or cisplatin 75 mg/m2 + pemetrexed 500 mg/m2 q3w.1
AUC=area under the curve; IV=intravenous; Pt=platinum.

Safety Data
View a selected safety profile of adverse reactions seen in clinical trials.

Dosing Schedules
Find dosing information to get patients started on therapy.
More NSCLC Indications
Learn how OPDIVO and OPDIVO-based combinations treat non-small cell lung cancer.
References:
- OPDIVO [package insert]. Princeton, NJ: Bristol-Myers Squibb Company.
- Carbone DP, Ciuleanu TE, Cobo M, et al. Nivolumab plus ipilimumab with chemotherapy as first-line treatment for patients with metastatic non-small-cell lung cancer: final, 6-year outcomes from CheckMate 9LA. ESMO Open 2025. Doi + TK.
- YERVOY [package insert]. Princeton, NJ: Bristol-Myers Squibb Company.
- Reck M, Ciuleanu T-E, Cobo M, et al. First-line nivolumab plus ipilimumab with two cycles of chemotherapy versus chemotherapy alone (four cycles) in advanced non-small cell lung cancer. CheckMate 9LA 2-year update. ESMO Open. 2021;6(5):100273.
- Data on file. NIVO 566. Princeton, NJ: Bristol-Myers Squibb Company; 2020.
- Paz-Ares L, Ciuleanu T-E, Cobo M, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(2):198-211.
- Data on file. NIVO 562. Princeton, NJ: Bristol-Myers Squibb Company; 2020.
- Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Non-Small Cell Lung Cancer. V.5.2025. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. Accessed June 20, 2025. To view the most recent and complete version of the guideline, go to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibilities for their application or use in any way.





