OPDIVO Qvantig™ (nivolumab + hyaluronidase-nvhy) is approved as a subcutaneous injection in the maintenance phase of treatment
Unresectable or Metastatic Melanoma
With 10 years of follow-up in Checkmate 067
A chance for long-term survival in patients with metastatic melanoma1-3

INDICATION OPDIVO® (nivolumab), in combination with YERVOY® (ipilimumab), is indicated for the treatment of adult and pediatric patients 12 years of age and older with unresectable or metastatic melanoma.
CHECKMATE 067: METASTATIC MELANOMA
A 3-arm, phase 3 study in the first-line treatment of metastatic melanoma1,2

- The primary endpoints compared OPDIVO + YERVOY with YERVOY, and OPDIVO monotherapy with YERVOY1,4
Key exclusion criteria1
- Patients with active brain metastasis, ocular melanoma, autoimmune disease, a medical condition requiring systemic treatment with corticosteroids (more than 10 mg daily prednisone equivalent) or other immunosuppressive medication within 14 days of the start of study therapy, a positive result for hepatitis B or C, and a history of HIV1
*The recommended dose of OPDIVO is 1 mg/kg administered as an intravenous infusion over 30 minutes, followed by YERVOY 3 mg/kg administered as an intravenous infusion over 30 minutes on the same day, q3w for a maximum of 4 doses or until unacceptable toxicity, whichever occurs earlier. After completing 4 doses of the combination, administer OPDIVO as a single agent, either 240 mg q2w or 480 mg q4w, administered as an intravenous infusion over 30 minutes until disease progression or unacceptable toxicity. Review the Prescribing Information for YERVOY for additional information prior to initiation.1 Please refer to the Prescribing Information for dosing in pediatric patients age 12 years and older and weighing less than 40 kg.1
AJCC=American Joint Committee on Cancer; DOR=duration of response; HIV=human immunodeficiency virus; IV=intravenous; M=metastasis; ORR=overall response rate; OS=overall survival; PD-L1=programmed death-ligand 1; PFS=progression-free survival; q2w=every 2 weeks; q3w=every 3 weeks; q4w=every 4 weeks.
43% of ITT patients were still alive at 10 years2,3
ITT POPULATION: OVERALL SURVIVAL ANALYSIS AT 10 YEARS2,3
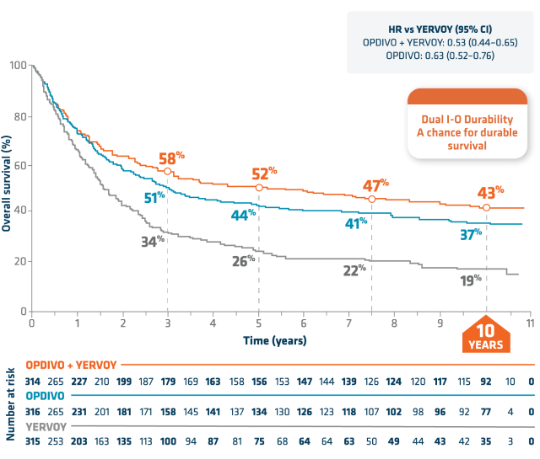
Median OS reached at 6 years2,3
In ITT patients (95% CI, months)1:
- ITT HR vs YERVOY at primary analysis of
28 months:- OPDIVO + YERVOY: 0.55 (0.44–0.69)*; P<0.0001†‡
- OPDIVO: 0.63 (0.50–0.78)*; P<0.0001†‡
- mOS at 10 years (95% CI, months)2,3:
- OPDIVO + YERVOY: 71.9 (38.2–114.4)
- OPDIVO: 36.9 (28.2–58.7)
- YERVOY: 19.9 (16.8–24.6)
This study was not designed to compare OPDIVO + YERVOY with OPDIVO.
*Based on a stratified proportional hazards model.1
†Based on stratified log-rank test.1
‡If the maximum of the two OS p-values is less than 0.04 (a significance level by the Hochberg procedure), then both p-values are considered significant.1
CI=confidence interval; HR=hazard ratio; I-O=immuno-oncology; ITT=intent to treat; mOS=median OS; OS=overall survival; NR=not reached.
31% of ITT patients were still progression-free at 10 years3
ITT POPULATION: PFS ANALYSIS AT 10 YEARS3

In ITT patients (95% CI, months)1:
- mPFS at primary analysis of 9 months:
- OPDIVO + YERVOY: 11.5 (8.9–16.7)
- OPDIVO: 6.9 (4.3–9.5)
- YERVOY: 2.9 (2.8–3.4)
- HR vs YERVOY:
- OPDIVO + YERVOY: 0.42 (0.34–0.51)*; P<0.0001†‡
- OPDIVO: 0.57 (0.47–0.69)*; P<0.0001†‡
In ITT population at 10 years (95% CI)3:
- mPFS at 10 years:
- OPDIVO + YERVOY: 11.5 (8.9–20.0)
- OPDIVO: 6.9 (5.1–10.2)
- YERVOY: 2.9 (2.8–3.1)
PFS was a co-primary endpoint.1
*Based on a stratified proportional hazards model.1
†Based on stratified log-rank test.1
‡P-value is compared with .005 of the allocated alpha for final PFS treatment comparisons.1
mPFS=median progression-free survival.
Select Important Safety Information
Serious Adverse Reactions
In Checkmate 067, serious adverse reactions (74% and 44%), adverse reactions leading to permanent discontinuation (47% and 18%) or to dosing delays (58% and 36%), and Grade 3 or 4 adverse reactions (72% and 51%) all occurred more frequently in the OPDIVO plus YERVOY arm (n=313) relative to the OPDIVO arm (n=313). The most frequent (≥10%) serious adverse reactions in the OPDIVO plus YERVOY arm and the OPDIVO arm, respectively, were diarrhea (13% and 2.2%), colitis (10% and 1.9%), and pyrexia (10% and 1.0%).
Common Adverse Reactions
In Checkmate 067, the most common (≥20%) adverse reactions in the OPDIVO plus YERVOY arm (n=313) were fatigue (62%), diarrhea (54%), rash (53%), nausea (44%), pyrexia (40%), pruritus (39%), musculoskeletal pain (32%), vomiting (31%), decreased appetite (29%), cough (27%), headache (26%), dyspnea (24%), upper respiratory tract infection (23%), arthralgia (21%), and increased transaminases (25%). In Checkmate 067, the most common (≥20%) adverse reactions in the OPDIVO arm (n=313) were fatigue (59%), rash (40%), musculoskeletal pain (42%), diarrhea (36%), nausea (30%), cough (28%), pruritus (27%), upper respiratory tract infection (22%), decreased appetite (22%), headache (22%), constipation (21%), arthralgia (21%), and vomiting (20%).
Please see additional Important Safety Information below.
ITT population: At 10 years, confirmed overall response rate5

Confirmed ORR in ITT patients at 9-month primary analysis (95% CI)1:
- OPDIVO + YERVOY: 50% (44–55); P<0.0001,† CR: 9%, PR: 41%
- OPDIVO: 40% (34–46); P<0.0001,† CR: 9%, PR: 31%
- YERVOY: 14% (10–18); CR: 2%, PR: 12%
ITT response analysis at 10 years (95% CI) in the OPDIVO arm5:
- ORR (n=132/316): 42% (36–47); CR: 17%, PR: 25%
OPDIVO mDOR at 10 years: 103 mos (46–NR)5
ORR was a secondary endpoint.1
*Percentages may not total 100 after rounding.
†Based on the stratified Cochran-Mantel-Haenszel test.1
BOR=best overall response; CR=complete response; mDOR=median duration of response; NA=not available; PR=partial response.
Induction with intravenous OPDIVO + YERVOY followed by OPDIVO Qvantig injection in the monotherapy maintenance phase1,6,7
OPDIVO is still available as an IV infusion in the monotherapy maintenance phase. View OPDIVO IV dosing schedule >
For patients age 12 and older and weighing ≥40 kg and patients 12 and older weighing less than 40 kg, please refer to the Prescribing Information.

INDICATION OPDIVO Qvantig (nivolumab + hyaluronidase-nvhy), as monotherapy, is indicated for the treatment of adult patients with unresectable or metastatic melanoma following treatment with intravenous nivolumab and ipilimumab combination therapy.
Limitations of Use: OPDIVO Qvantig is not indicated in combination with ipilimumab for the treatment of unresectable or metastatic melanoma.
As of February 25, 2022, the infusion time for YERVOY was updated from 90 minutes to 30 minutes when used as a monotherapy or in combination with OPDIVO in metastatic melanoma1
OPDIVO Qvantig is administered as a 3- to 5-minute subcutaneous injection7
OPDIVO is administered as a 30-minute IV infusion1
The first dose of OPDIVO Qvantig monotherapy should be administered after completing up to a maximum of 4 doses of the OPDIVO and YERVOY combination therapy1,7
Review the Full US Prescribing Information for OPDIVO, OPDIVO Qvantig, and YERVOY for recommended dosage information
- No premedication required with OPDIVO + YERVOY or OPDIVO Qvantig1,6,7

Safety Data
View a selected safety profile of adverse reactions seen in clinical trials.

Dosing Schedules
Find dosing information to get patients started on therapy.

More Melanoma Options
Learn more about all melanoma indications.
Discover another option for patients with unresectable or metastatic melanoma
See OPDIVO + YERVOY dual I-O efficacy data in multiple tumor types
References:
- OPDIVO [package insert]. Princeton, NJ: Bristol-Myers Squibb Company.
- Larkin J, Chiarion-Sileni V, Gaudy-Marqueste C, et al. 10-Year survival outcomes from the phase 3 CheckMate 067 trial of nivolumab plus ipilimumab in advanced melanoma. Oral presentation at: ESMO Congress 2024; September 13-17, 2024; Barcelona, Spain.
- Wolchok JD, Chiarion-Sileni V, Rutkowski P, et al. Final, 10-year outcomes with nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. Published online September 15, 2024. doi:10.1056/NEJMoa2407417
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381 (16):1535-1546.
- Wolchok JD, Chiarion-Sileni V, Rutkowski P, et al. Final, 10-year outcomes with nivolumab plus ipilimumab in advanced melanoma (Suppl.). N Engl J Med. Published online September 15, 2024.
- YERVOY [package insert]. Princeton, NJ: Bristol-Myers Squibb Company.
- OPDIVO Qvantig [package insert]. Princeton, NJ: Bristol-Myers Squibb Company.





