1L mNSCLC (PD-L1 ≥1%)
Chemo-Free Option
With durable survival at 6 years*
Set out towards a future dual I-O can deliver1,2†‡
Longest ever reported follow-up of any approved 1L I-O in a phase 3 mNSCLC trial (median follow-up was 78.8 months)
*vs chemo.1
†In Checkmate 227, patients received platinum-doublet chemo q3w; NSQ: pemetrexed + carboplatin or cisplatin; SQ: gemcitabine + carboplatin or cisplatin.1
‡For patients with no EGFR or ALK tumor aberrations.1

INDICATION OPDIVO® (nivolumab), in combination with YERVOY® (ipilimumab), is indicated for the first-line treatment of adult patients with metastatic non-small cell lung cancer (NSCLC) whose tumors express PD-L1 (≥1%) as determined by an FDA-approved test, with no EGFR or ALK genomic tumor aberrations.
CHECKMATE 227: IN A CROSS-HISTOLOGY TRIAL FOR PATIENTS WITH mNSCLC (PD-L1 ≥1%)
Reset expectations: Durable survival with OPDIVO® + YERVOY®: 22% of patients alive at 6 years2*
OS FOR PD-L1 ≥1% (EXTENDED FOLLOW-UP ANALYSIS)1-3

Between 2007 and 2013, prior to I-O approval,
the 5-year relative survival rate for mNSCLC patients was 5%4†
Minimum/median follow-up for OS: 73.5/78.8 months.2
Median PFS, with a minimum follow-up of 73.5 months, was 5.1 months (95% CI: 4.1–6.3) with OPDIVO + YERVOY and 5.6 months (95% CI: 4.6–5.8) with chemo; 0.80 (95% CI: 0.68–0.94)2,5‡
29% of patients enrolled had SQ disease; 71% had NSQ disease1
The only I-O combination with mDOR of 24.5 months among responders2‡
ORR, with a minimum follow-up of 73.5 months, was 36% (n=144/396; CR=6.8%, PR=29.5%) with OPDIVO + YERVOY and 30% (n=118/397; CR=2.0%, PR=27.7%) with chemo2,5
mDOR, with a minimum follow-up of 73.5 months, was 24.5 months (95% CI: 15.5–34.5) with OPDIVO + YERVOY and 6.7 months (95% CI: 5.6–7.6) with chemo2
Study design: Checkmate 227 was a randomized, open-label, phase 3 trial in patients with metastatic or recurrent NSCLC. Key eligibility criteria included patients 18 years or older, stage IV or recurrent NSCLC, ECOG PS 0/1, and no prior systemic anticancer therapy. Patients with known EGFR mutations or ALK translocations sensitive to available targeted inhibitor therapy, untreated brain metastases, carcinomatous meningitis, active autoimmune disease, or medical conditions requiring systemic immunosuppression were excluded from the study. Treatment continued until disease progression, unacceptable toxicity, or for up to 24 months. Tumor specimens were evaluated prospectively using the PD-L1 IHC 28-8 pharmDx assay at a central laboratory. In Part 1a (n=793), patients with PD-L1 ≥1% were randomized to either OPDIVO 3 mg/kg q2w§ + YERVOY 1 mg/kg q6w (n=396) or platinum-doublet chemotherapy* (n=397). The primary endpoint in Part 1a was OS in patients with PD-L1 ≥1%. Pre-specified descriptive efficacy measures included PFS, ORR, and DOR.1,6
*vs chemo. In Checkmate 227, patients in the comparator arm received up to 4 cycles of platinum-doublet chemo q3w; NSQ: pemetrexed + carboplatin or cisplatin, with optional pemetrexed maintenance following chemo; SQ: gemcitabine + carboplatin or cisplatin.1,7
†Data sourced from the SEER database.4
‡In Checkmate 227 Part 1a, PFS, ORR, and DOR were pre-specified descriptive analyses. The primary efficacy outcome measure was OS.1,7
§The recommended dosage of OPDIVO per the Prescribing Information is 360 mg q3w with YERVOY 1 mg/kg q6w.1
1L=first-line; ALK=anaplastic lymphoma kinase; CI=confidence interval; CR=complete response; DOR=duration of response; ECOG PS=Eastern Cooperative Oncology Group Performance Status; EGFR=epidermal growth factor receptor; HR=hazard ratio; IHC=immunohistochemistry; I-O=immuno-oncology; mDOR=median duration of response; mos=months; mNSCLC=metastatic non-small cell lung cancer; NSCLC=non-small cell lung cancer; NSQ=non-squamous; ORR=overall response rate; OS=overall survival; PD-L1=programmed death-ligand 1; PFS=progression-free survival; PR=partial response; q2w=every 2 weeks; q3w=every 3 weeks; q6w=every 6 weeks; SEER=Surveillance, Epidemiology, and End Results; SQ=squamous; TTR=time to response.
Select Important Safety Information
Serious Adverse Reactions
In Checkmate 227, serious adverse reactions occurred in 58% of patients (n=576). The most frequent (≥2%) serious adverse reactions were pneumonia, diarrhea/colitis, pneumonitis, hepatitis, pulmonary embolism, adrenal insufficiency, and hypophysitis. Fatal adverse reactions occurred in 1.7% of patients; these included events of pneumonitis (4 patients), myocarditis, acute kidney injury, shock, hyperglycemia, multi-system organ failure, and renal failure.
Common Adverse Reactions
In Checkmate 227, the most common (≥20%) adverse reactions were fatigue (44%), rash (34%), decreased appetite (31%), musculoskeletal pain (27%), diarrhea/colitis (26%), dyspnea (26%), cough (23%), hepatitis (21%), nausea (21%), and pruritus (21%).
Please see additional Important Safety Information below.
National Comprehensive Cancer Network® (NCCN®) Category 1 recommended8
Nivolumab (OPDIVO) + ipilimumab (YERVOY)
NCCN Category 1, other recommended
PD-L1 1%-49%
NCCN Category 1, useful in certain circumstances
PD-L1 ≥50%
- Nivolumab (OPDIVO) + ipilimumab (YERVOY) is recommended as a Category 1, other recommended, first-line therapy option for eligible patients with metastatic NSCLC with PD-L1 1%–49%, or as useful in certain circumstances, first-line therapy option for eligible patients with metastatic NSCLC with PD-L1 ≥50%, and performance status 0–2 (V.11.2024), including those who are EGFR,* ALK, ROS1, BRAF V600E, NTRK1/2/3, METex14, and RET negative, and with no contraindications to PD-1 or PD-L1 inhibitors8
*EGFR exon 19 deletion, exon 21 L858R, EGFR S768I, L861Q, and/or G719X mutation.8
Please see updated NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for a complete listing of all NCCN-recommended agents, including preferred options. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
NCCN=National Comprehensive Cancer Network® (NCCN®); PD-1=programmed cell death protein-1.
OPDIVO + low-dose YERVOY (1 mg/kg)-based dosing1*
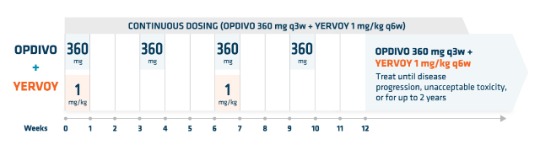
OPDIVO is administered as an IV infusion over 30 minutes1
YERVOY is administered as an IV infusion over 30 minutes9
*The previously approved dosing of OPDIVO 3 mg/kg q2w was based on the pivotal trial Checkmate 227. The recommended dosage of OPDIVO per the Prescribing Information is 360 mg q3w with YERVOY 1 mg/kg q6w, administered as an IV infusion over 30 minutes until disease progression, unacceptable toxicity, or for up to 2 years.1
IV=intravenous.

Safety Data
View a selected safety profile of adverse reactions seen in clinical trials.

Dosing Schedules
Find dosing information to get patients started on therapy.
More NSCLC Indications
Learn how OPDIVO and OPDIVO-based combinations treat non-small cell lung cancer.
See OPDIVO + YERVOY dual I-O efficacy data in multiple tumor types
References:
- OPDIVO [package insert]. Princeton, NJ: Bristol-Myers Squibb Company.
- Ramalingam S, Ciuleanu TE, Bernabe Caro R, et al. Six-year survival and health-related quality of life outcomes with first-line nivolumab plus ipilimumab in patients with metastatic NSCLC from CheckMate 227. Oral presentation at WCLC 2023. Abstract OA14.03.
- Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med. 2019;381(21):2020-2031.
- American Cancer Society. Cancer facts & figures. Cancer.org. 2018. Accessed May 24, 2022. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2018/cancer-facts-and-figures-2018.pdf
- Data on file. BMS-REF-NIVO-0244. Princeton, NJ: Bristol-Myers Squibb Company; 2023.
- Brahmer JR, Lee JS, Ciuleanu TE, et al. Five-year survival outcomes with nivolumab plus ipilimumab versus chemotherapy as first-line treatment for metastatic non-small cell lung cancer in CheckMate 227. J Clin Oncol. 2023;41(6):1200-1212.
- Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med. 2019;381(21):2020-2031 [supplementary appendix].
- Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Non-Small Cell Lung Cancer. V.11.2024. ©National Comprehensive Cancer Network, Inc. 2024. All rights reserved. Accessed September 24, 2024. To view the most recent and complete version of the guidelines, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
- YERVOY [package insert]. Princeton, NJ: Bristol-Myers Squibb Company.





