| OPDIVO Qvantig + platinum-doublet chemotherapy |
|---|
OPDIVO Qvantig™ (nivolumab + hyaluronidase-nvhy) is approved as a subcutaneous injection
Dosing schedules

Find dosing information to get patients with metastatic/recurrent or early-stage NSCLC started on therapy
Neoadjuvant Treatment of Resectable NSCLC1,2
INDICATIONS
INTRAVENOUS USE OPDIVO® (nivolumab), in combination with platinum-doublet chemotherapy, is indicated as neoadjuvant treatment of adult patients with resectable (tumors ≥4 cm or node positive) non-small cell lung cancer (NSCLC). View OPDIVO IV dosing schedule >
SUBCUTANEOUS USE OPDIVO Qvantig, in combination with platinum-doublet chemotherapy, is indicated as neoadjuvant treatment of adult patients with resectable (tumors ≥4 cm or node positive) non-small cell lung cancer (NSCLC).
| DOSING & SCHEDULE | DURATION |
|---|---|
| 900 mg nivolumab and 15,000 units hyaluronidase subcutaneous injection over 3-5 minutes with platinum-doublet chemotherapy on the same day q3w | In combination with platinum-doublet chemotherapy for 3 cycles |
No premedications are required with OPDIVO Qvantig.
Platinum-doublet chemotherapy q3w for 3 cycles: Any histology: paclitaxel and carboplatin; NSQ: pemetrexed and cisplatin or carboplatin; SQ: gemcitabine and cisplatin.
Review the U.S. Full Prescribing Information for OPDIVO Qvantig.
Perioperative Treatment of Resectable NSCLC1,2
INDICATIONS
INTRAVENOUS USE OPDIVO, in combination with platinum-doublet chemotherapy, is indicated for the neoadjuvant treatment of adult patients with resectable (tumors ≥4 cm or node positive) NSCLC and no known epidermal growth factor receptor (EGFR) mutations or anaplastic lymphoma kinase (ALK) rearrangements, followed by single-agent OPDIVO as adjuvant treatment after surgery. View OPDIVO IV dosing schedule >
SUBCUTANEOUS USE OPDIVO Qvantig, in combination with platinum-doublet chemotherapy, is indicated for the neoadjuvant treatment of adult patients with resectable (tumors ≥4 cm or node positive) NSCLC and no known epidermal growth factor receptor (EGFR) mutations or anaplastic lymphoma kinase (ALK) rearrangements, followed by OPDIVO Qvantig as monotherapy in the adjuvant setting after surgical resection.
| Neoadjuvant: OPDIVO Qvantig + platinum-doublet chemotherapy |
|---|
| DOSING & SCHEDULE | DURATION |
|---|---|
| 900 mg nivolumab and 15,000 units hyaluronidase subcutaneous injection over 3-5 minutes with platinum-doublet chemotherapy on the same day q3w | In combination with platinum-doublet chemotherapy until disease progression or unacceptable toxicity, for up to 4 cycles |
| Adjuvant: OPDIVO Qvantig Monotherapy |
|---|
| DOSING & SCHEDULE | DURATION |
|---|---|
| 1,200 mg nivolumab and 20,000 units hyaluronidase subcutaneous injection over 3-5 minutes q4w | Following neoadjuvant therapy and surgery, administer as a single agent until disease progression, recurrence, or unacceptable toxicity, for up to 13 cycles (up to 1 year) |
No premedications are required with OPDIVO Qvantig.
Platinum-doublet chemotherapy q3w for up to 4 cycles: Any histology: paclitaxel and carboplatin; NSQ: pemetrexed and cisplatin or carboplatin; SQ: cisplatin and docetaxel.
Review the U.S. Full Prescribing Information for OPDIVO Qvantig.
1L Metastatic Non-Small Cell Lung Cancer (PD-L1 ≥1%)1
INDICATION
INTRAVENOUS USE OPDIVO, in combination with YERVOY® (ipilimumab), is indicated for the first-line treatment of adult patients with metastatic non-small cell lung cancer (NSCLC) whose tumors express PD-L1 (≥1%) as determined by an FDA-approved test, with no EGFR or ALK genomic tumor aberrations.*
*Information on FDA-approved tests for the determination of PD-L1 expression in NSCLC is available at http://www.fda.gov/CompanionDiagnostics.
| OPDIVO + YERVOY†‡ |
|---|
| DOSING & SCHEDULE | DURATION |
|---|---|
| 360 mg of OPDIVO IV infusion over 30 minutes q3w WITH 1 mg/kg of YERVOY IV infusion over 30 minutes q6w |
In combination with ipilimumab until disease progression, unacceptable toxicity, or up to 2 years in patients without disease progression |
No premedications are required with OPDIVO and YERVOY.
†When OPDIVO is administered in combination with YERVOY, if OPDIVO is withheld or discontinued, YERVOY should also be withheld or discontinued.1
‡Interrupt or slow the rate of infusion in patients with mild or moderate infusion-related reactions. Discontinue OPDIVO or OPDIVO + YERVOY in patients with severe or life-threatening infusion-related reactions.1
Review the U.S. Full Prescribing Information for OPDIVO and YERVOY.
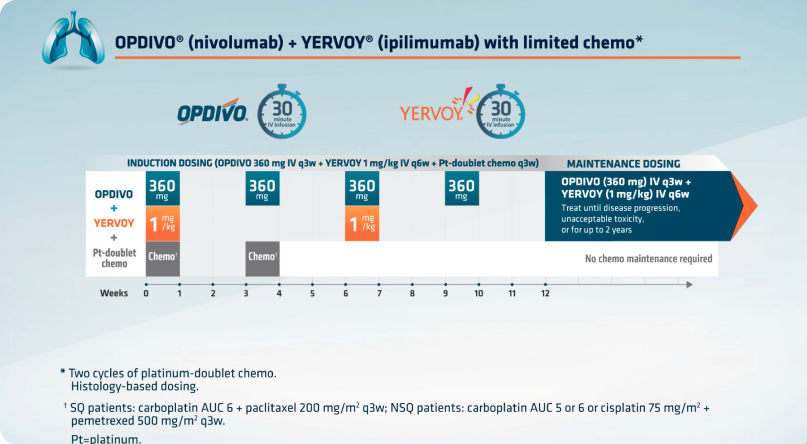
1L Metastatic or Recurrent Non-Small Cell Lung Cancer1
INDICATION
INTRAVENOUS USE OPDIVO, in combination with YERVOY and 2 cycles of platinum-doublet chemotherapy, is indicated for the first-line treatment of adult patients with metastatic or recurrent non-small cell lung cancer (NSCLC), with no EGFR or ALK genomic tumor aberrations.
| OPDIVO + YERVOY with 2 cycles of histology-based platinum-doublet chemotherapy |
|---|
| DOSING & SCHEDULE*† | DURATION |
|---|---|
| OPDIVO + YERVOY | |
| 360 mg of OPDIVO IV infusion over 30 minutes q3w WITH 1 mg/kg of YERVOY IV infusion over 30 minutes q6w |
In combination with ipilimumab until disease progression, unacceptable toxicity, or up to 2 years in patients without disease progression |
| AND platinum-doublet chemotherapy | |
| Histology-based platinum-doublet chemotherapy q3w |
2 cycles of histology-based platinum-doublet chemotherapy |
Histology-based chemo; SQ patients: carboplatin AUC 6 + paclitaxel 200 mg/m2 q3w; NSQ patients: carboplatin AUC 5 or 6 or cisplatin 75 mg/m2 + pemetrexed 500 mg/m2 q3w with optional pemetrexed maintenance therapy.
For the r/m NSCLC dosing regimen in combination with chemo: on the first week, 4 agents will be administered (OPDIVO 360 mg + YERVOY 1 mg/kg + histology-based chemo), followed by 3 agents (OPDIVO + histology-based chemo) on the third week, 2 agents (OPDIVO + YERVOY) on the sixth week, and OPDIVO monotherapy on the ninth week, followed by maintenance therapy of OPDIVO + YERVOY.
No premedications are required with OPDIVO and YERVOY.
*When OPDIVO is administered in combination with YERVOY, if OPDIVO is withheld or discontinued, YERVOY should also be withheld or discontinued.1
†Interrupt or slow the rate of infusion in patients with mild or moderate infusion-related reactions. Discontinue OPDIVO or OPDIVO + YERVOY in patients with severe or life-threatening infusion-related reactions.1
Review the U.S. Full Prescribing Information for OPDIVO and YERVOY.
2L Metastatic Non-Small Cell Lung Cancer1,2
INDICATIONS
INTRAVENOUS USE OPDIVO is indicated for the treatment of adult patients with metastatic non-small cell lung cancer (NSCLC) with progression on or after platinum-based chemotherapy. Patients with EGFR or ALK genomic tumor aberrations should have disease progression on FDA-approved therapy for these aberrations prior to receiving OPDIVO. View OPDIVO IV dosing schedule >
SUBCUTANEOUS USE OPDIVO Qvantig, as monotherapy, is indicated for the treatment of adult patients with metastatic non-small cell lung cancer (NSCLC) with progression on or after platinum-based chemotherapy. Patients with EGFR or ALK genomic tumor aberrations should have disease progression on FDA-approved therapy for these aberrations prior to receiving OPDIVO Qvantig.
Limitations of Use: OPDIVO Qvantig is not indicated in combination with ipilimumab for the treatment of metastatic NSCLC.
| OPDIVO Qvantig Monotherapy |
|---|
| DOSING & SCHEDULE | DURATION |
|---|---|
600 mg nivolumab and 10,000 units hyaluronidase subcutaneous injection over 3-5 minutes q2w OR 1,200 mg nivolumab and 20,000 units hyaluronidase subcutaneous injection over 3-5 minutes q4w |
Until disease progression or unacceptable toxicity |
No premedications are required with OPDIVO Qvantig.
Review the U.S. Full Prescribing Information for OPDIVO Qvantig.
1L=first-line; 2L=second-line; ALK=anaplastic lymphoma kinase; AUC=area under the curve; EGFR=epidermal growth factor receptor; FDA=U.S. Food and Drug Administration; IV=intravenous; NSQ=nonsquamous; r/m=recurrent or metastatic; PD-L1=programmed death-ligand 1; q2w=every 2 weeks; q3w=every 3 weeks; q4w=every 4 weeks; q6w=every 6 weeks; r/m NSCLC=recurrent or metastatic non-small cell lung cancer; SQ=squamous.

Treatment Modifications
See recommended dosing modifications for immune-mediated adverse reactions with OPDIVO, YERVOY, or OPDIVO Qvantig.

Preparation and Administration
Find instructions for preparation, administration, and storage for both OPDIVO infusions and OPDIVO Qvantig subcutaneous injections.

Dosing Guide
A guide to dosing intravenous OPDIVO and subcutaneous injection OPDIVO Qvantig.
References:
- OPDIVO [package insert]. Princeton, NJ: Bristol-Myers Squibb Company.
- OPDIVO Qvantig [package insert]. Princeton, NJ: Bristol-Myers Squibb Company.





